HOWARTH LAB

SOCIAL
ADDRESS
Department of Pharmacology,
University of Cambridge,
Tennis Court Road,
Cambridge
CB2 1PD
UK
CONTACT
e: mh2186@cam.ac.uk
t: 01223 334176
2026 Mark Howarth. All rights reserved.
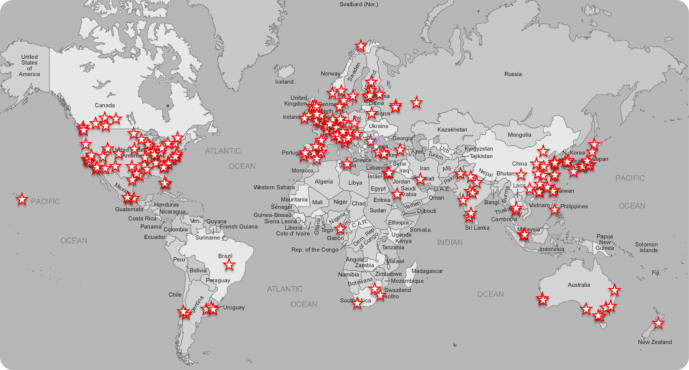
We have distributed the resources developed in the lab to 49 countries:
Please say hello on BlueSky @HowarthSci when you receive one of our reagents.
Purified Protein Availability
Protein for SpyCatcher003, Cys-SpyCatcher003, SpyTag003-MBP and traptavidin are distributed not-for-profit at Kerafast.
Certain reagents are also commercialised through Bio-Rad.
Controlled valency streptavidins are available from Abcam or Kromnigon.
Plasmids
The following plasmids were given to the plasmid repository Addgene for easy distribution to academic laboratories. Addgene have
shipped at least 3,000 samples from us. Non-academics should contact Mark for information.
Feel free to e-mail for comments or advice on these systems.
(a) Biotin binding tools
(i) Traptavidin, a mutant of streptavidin with lower off-rate, increased thermostability, and greater mechanical strength.
Also Traptavidin-E6 (for ion-exchange chromatography purification)
(ii) Monovalent streptavidin and cis or trans Divalent streptavidin:
Alive-H6 (biotin binding with 6 histidines for Ni-NTA purification)
Alive-E6 (biotin binding with 6 glutamates for ion-exchange purification)
Dead (streptavidin subunit with no biotin binding)
Dead-Aspartate loop (streptavidin subunit with no biotin binding and negatively charged loop for ion-exchange chromatography
resolution)
(J Mol Biol 2014 and Nature Methods 2006)
(iii) SpyAvidin subunits: see below at (b)(iii)
(iv) Biotinylation tools
pDisplay-BirA-ER for biotinylation in the mammalian secretory pathway
pDisplay-AP-CFP-TM as a target for BirA in mammalian cells
BirA-His6 for bacterial overexpression
(GST-BirA for bacterial overexpression- please request from Chris O’Callaghan)
(v) Fluorophore-friendly streptavidin
K121R streptavidin or Flavidin plasmids
(b) Isopeptide tools
See our tips on SpyTag use here.
Also, please see a hyperlinked bibliography of all papers using Spy and Snoop tools here.
SpyTag003 cloning NOTE! If SpyTag is positioned right next to the initiation codon, with certain codon usage we found low induction in
bacteria. This is probably because of secondary structure formation with vector-derived sequences in the mRNA. Including a GSS
spacer with the following codons worked well in pET28, with a T7 promoter (download insert sequence):
Ideally you should check your particular construct using an online Ribosome Binding Site calculator, with 10,000 representing a
satisfactory score.
(i) Standard SpyTag/SpyCatcher tools
SpyCatcher003 for irreversible peptide-protein ligation
SpyCatcher003 S49C for Cysteine-mediated anchoring or labelling
SpyCatcher003-sfGFP linked to superfolder GFP
SpyTag003-MBP for irreversible peptide-protein ligation
AviTag-SpyTag003-MBP combining irreversible ligation via SpyTag003 with a biotinylation tag for anchoring to streptavidin
SpyTag003-mKate2 linked to bright red fluorescent protein
SpyTag003-mClover3 linked to bright greeen fluorescent protein
SpyTag003-sfGFP linked to superfolder GFP
SpyTag-sfGFP (original SpyTag linked to superfolder GFP)
TfR-sfGFP-myc tag-SpyCatcher003 for mammalian cell-surface display
EGFP-Talin head-SpyCatcher003 for mammalian cytosolic expression
SpyTag003-Talin rod-mCherry for mammalian cytosolic expression
(Also earlier generations of these tools:
AviTag-SpyCatcher for anchoring on streptavidin-resin
SpyTag-MBP,
SpyCatcher,
SpyCatcher EQ as a non-reactive control for SpyCatcher
MBPx-SpyCatcher for anchoring on amylose-resin
SpyCatcher002 for accelerated irreversible peptide-protein ligation
AviTag-SpyCatcher002 for anchoring on streptavidin-resin
Cys-SpyCatcher002 for thiol-reactive anchoring or labeling
SpyTag002-MBP for accelerated irreversible peptide-protein ligation
SpyCatcher002 EQ as a non-reactive control)
(ii) Ligase and DogCatcher tools
AviTag-SnoopLigase for peptide-peptide ligation
HaloTag7-SnoopLigase for peptide-peptide ligation
SnoopTagJr-MBP for SnoopLigase peptide-peptide ligation or reaction with SnoopCatcher
SUMO-DogTag for SnoopLigase peptide-peptide ligation
IMX-DogTag for SnoopLigase-mediated oligomerization
DogCatcher for ligation to DogTag-partners
AviTag-DogCatcher-MBP for anchoring on streptavidin-resin
Cys-DogCatcher for thiol-reactive anchoring or labeling
DogCatcher-sfGFP
sfGFP with SpyTag003 in loop A or loop B or loop C
sfGFP with DogTag in loop A or loop B or loop C
AviTag-DogTag-MBP for anchoring on streptavidin-resin
HaloTag7SS-DogTag
SnoopTag2-AffiHER2 for an affibody to HER2 that can be ligated to DogTag2 or SnoopCatcher
AviTag-DogTag2-MBP for site-specific biotinylation and ligation to SnoopTag2 or DogCatcher
SnoopLigase2 for peptide-peptide ligation
TfR-sfGFP-SnoopTag2 for mammalian cell-surface display of SnoopTag2
HA-MBP-SnoopLigase2 N775Q-KDEL for peptide-peptide ligation in the mammalian ER
RBD-DogTag2-SpyTag encoding SARS-CoV-2 RBD for reaction with SnoopTag2 or DogCatcher
SUMO-DogTag2
HaloTag7-SnoopLigase2 for peptide-peptide ligation
SnoopTagJr-sfGFP
SnoopTag2-sfGFP (GenBank OQ923256, Addgene 201810)
TG2x-DogTag2, a variant of human Transglutaminase 2 for ligation to SnoopTag2 or DogCatcher
SnoopTag2-TGFα encoding human Transforming Growth Factor-α for ligation to DogTag2 or SnoopCatcher
(SnoopLigase2 or SnoopLigase is recommended over SpyLigase for higher efficiency)
(iii) SpyAvidin subunits:
Dead-SpyCatcher (DCatch)
Dead-SpyTag, (DTag)
Traptavidin-E6 (Tre)
Traptavidin (Tr)
(iv) SpyRings:
SpyTag-β-lactamase-SpyCatcher, for enzyme cyclization: through CPEC one can insert other enzymes in this scaffold
(v) Solid-phase polyproteam synthesis:
SnoopCatcher for irreversible peptide-protein ligation (an orthogonal pair to SpyTag/SpyCatcher)
SnoopTag-MBP for irreversible peptide-protein ligation (an orthogonal pair to SpyTag/SpyCatcher)
SpyCatcher-SnoopCatcher for bridging SpyTag and SnoopTag
MBPx-SpyCatcher for solid-phase anchoring on amylose-resin
AviTag-SpyCatcher for solid-phase anchoring on streptavidin-resin
SnoopTag-mEGFP-SpyTag, with BamHI sites for easy insertion of other proteins between SnoopTag and SpyTag
(vi) Biomaterials:
pQE80L TriCatcher-ELP, with two SpyCatchers and one SnoopCatcher
pQE80L TriCatcher-RGDSP, as above with an integrin-binding site
pQE80L TriCatcher-MMP, as above with an MMP cleavage site
pQE80L TriCatcher-RGDSP-MMP, with integrin-binding and MMP cleavage sites
pQE80L SpyTag-ELP-SpyTag
pQE80L SpyTag-ELP-SpyTag DK
(vii) Virus-like particle and oligomer assembly: (see note on protein availability above)
pET28a-SpyCatcher-mi3 (with C-tag)
pET28a-SpyCatcher-mi3 with His-tag (not described in Bruun et al. but may be more convenient for some labs and we can send Ni-
NTA protocol)
pET28a-SpyCatcher003-mi3 (with C-tag)
IMX-DogTag for SnoopLigase-mediated oligomerization
SpyCatcher002-oDi, coiled coil for dimerization
SpyCatcher002-oTri, coiled coil for trimerization
SpyCatcher002-oTet, coiled coil for tetramerization
SpyCatcher002-oPent, coiled coil for pentamerization
SpyCatcher002-oHex, coiled coil for hexamerization
SpyCatcher002-oHept, coiled coil for heptamerization
(vii) Affinity purification: (see note on protein availability above)
SpySwitch for capture and release of fusions to SpyTag, SpyTag002 or SpyTag003
SpyDock for capture and release of SpyTag- and SpyTag002-fusions (but SpySwitch is recommended)
(viii) SpyMask for bispecific binders
DoubleCatcher-ε-Lock
DoubleCatcher-δ-Lock
DoubleCatcher-γ-Lock
DoubleCatcher-β-Lock
DoubleCatcher-alpha-Lock
DoubleCatcher H-lock
DoubleCatcher
SpyCatcher003-TEVs-SpyTag003DA for protease-uncaging of reactivity
as well as various Fab, nanobody and affibody binders of HER2 linked to SpyTag
(ix) Antigens
Spy0469 (antigen from Streptococcus pyogenes)
PsaA (antigen from Streptococcus pneumoniae)
SpyTag003-OspC (antigen from Borrelia burgdorferi; Lyme Disease)
SpyTag-RBD (receptor-binding domain from Spike of SARS-CoV-2)
NDV HN-SpyTag (antigen from Newcastle Disease Virus)
SpyTag-RBD Omicron BQ.1.1
SpyTag-RBD Omicron XBB.1.5
SpyTag-RBD Delta
and multiviral quartets to elicit broad sarbecovirus antibody responses:
SpyTag-Quartet_NoLinker
SpyTag-Kraken Quartet
SpyTag-Quartet [SARS1]
SpyTag-Alternate Quartet
SpyTag-Quartet
Quartet-SpyTag
(x) Light control of isopeptide bond formation
TfR-SpyCatcher003(K31TAG)-sfGFP-MycTag-CTag
SpyCatcher003(K31TAG)-Talin rod-mScarletI
EGFP-Talin head-SpyCatcher003(K31TAG)
LifeAct-mNeonGreen -IRES- Talin head-SpyTag003
SpyTag003(M115A)-Talin rod-mCherry
SpyTag003(M115G)-Talin rod-mCherry
SpyTag003(V114T, V116T)-Talin rod-mCherry
(c) NeissLock
OAZ-GSY-SPM
ODC-Ctag
TGFa-GSY-SPM
(Of course, please enquire if there is a useful plasmid on which we have published, that is not listed here.)